What if you are hospitalized and the drug needed to save your life isn’t available? That possibility is less remote than you might think.
For the past two decades the US has been experiencing shortages of cancer drugs, antibiotics and even saline, a drug potentially needed by almost every patient who gets admitted to the hospital. Nearly all thirty of the most frequently used emergency department drugs experienced shortages from 2006-2019.
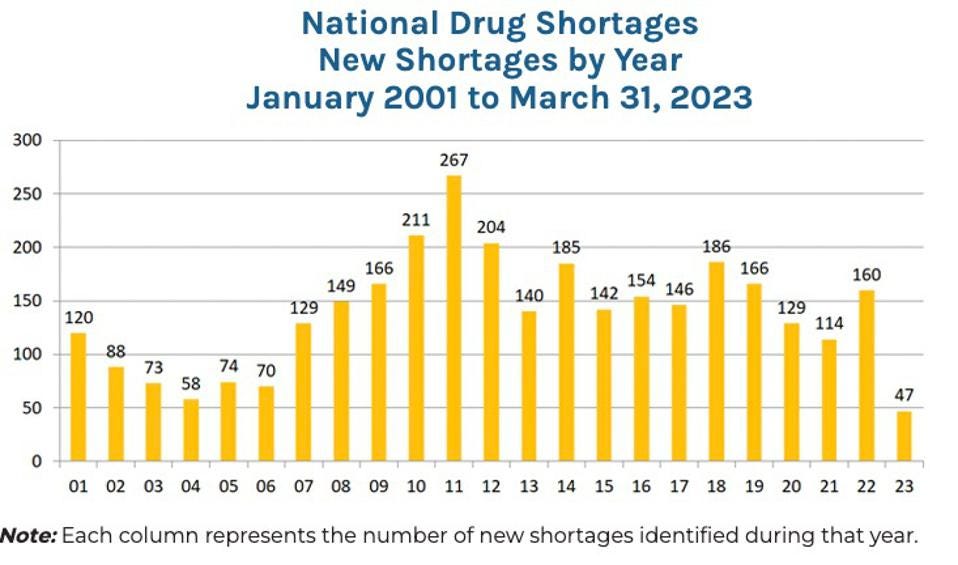
The graph below shows that the number of new drug shortages occurring each year has been a serious problem for the past two decades. At the end of 2022, there were 295 active drug shortages. While the average drug shortage lasts about 1.5 years, 15 critical drugs have been in shortage for more than a decade.
How can this happen in a country with a sophisticated and well-funded health-care system?
Most of the drugs in short supply are generics—drugs that are less-expensive copies of patented drugs. Most have been off-patent for an average of three decades. They have long since been approved by the Federal Food and Drug Administration (FDA). They are known to be safe and effective. Many cost less than $10 a pill.
When he was a White House advisor during the Obama administration, oncologist Ezekiel Emanuel estimated that about 10 percent of shortages of cancer drugs are due to a lack of raw materials needed to manufacture them.
A more important source of the problem is government policy.
Problem: the suppression of normal market forces. FDA regulations limit the ability of drug makers to advertise or otherwise communicate improvements in safety, reliability or efficacy to potential customers. This is on the theory that all generics are alike, so the only thing that matters is price.
These regulations remove the economic incentives to solve problems the way they would be solved in any normal market. Reliability of supply is a valuable thing. Buyers would pay something to assure it. But in the market for generic drugs, they are not given that opportunity.
Furthermore, the pharmacists who fill the prescriptions are implicitly or explicitly required to fill them with the generic that has the lowest price. The result: There is no competition by drug manufacturers on any product feature other than price.
It costs money to keep inventories high to assure the availability of supply. It also costs money to improve drug safety and to improve the efficacy of a drug—even a generic drug—in combatting diseases and saving lives. If manufacturers cannot recoup their investments to make these improvements, they will not be able to afford to make them.
Brand-name drug companies rely on foreign manufacturer—often in Europe or Puerto Rico to produce their medicines. The choice of a host country undoubtedly reflects the concern those companies have for safety and quality. In the generic market, by contrast, the race to the bottom has shifted production to the countries with the lowest manufacturing cost in the world—China and India.
In the United States, the FDA can inspect a manufacturing plant any day of the week, at the drop of a hat, with no prior warning. That doesn’t happen in China and India. So, we are holding manufacturers in the U.S. to a higher safety standard. But they cannot advertise that fact. Nor can they imply that because a drug is produced in the U.S., it is “probably” safer and of higher quality—although it probably is. In general, when consumers buy a drug at the local pharmacy, they have no way of knowing or even finding out where the drug (or its active ingredients) were produced.
In contrast, consider what happens in the largely unregulated market for aspirin. Aspirin is a generic product that does not fall under FDA regulation because it was grandfathered when the FDA was created. Because aspirin is sold directly to consumers (rather than through a pharmacist middleman), there can be different prices for different brands and producer reputation matters to many consumers.
When is the last time you noticed a shortage of aspirin or any other over-the-counter pain relief drug?
Problem: Unwise Output Regulations: When the FDA inspects a manufacturing plant, it uses a binary (pass/fail) system that does not reward companies for exceeding minimum required standards or using best practices to minimize production problems. And regulations make it difficult for competitors to take up the slack. If a shortage develops because the FDA shuts down a competitor’s plant, for example, the competitor must seek FDA approval before it can increase its output.
Problem: Medicare Part B Price Controls. Medicare Part B (which covers drugs administered on-site) allows doctors and hospitals to charge a small percent over the drug’s average selling price to cover the cost of administration. However, that “average selling price” is calculated across all manufacturers and is based on historical prices.
If a manufacturer sees a shortage developing and raises the price of its drug, the health care providers that are potential buyers it may actually lose money if they purchase it.
Problem: 340B Price Controls. This program forces manufacturers to sell drugs at a discount to hospitals and clinics treating low-income and uninsured patients. But hospitals are allowed to turn around and sell those same drugs to fully insured patients at list prices.
Hospitals and clinics have gained from these discounts—$6 billion in 2015. But there appears to be no gain for either patients or taxpayers. According to a study in the New England Journal of Medicine, there is no clear evidence of “expanded care or lower mortality among low-income patients.” At the same time, these price controls, like other price controls, lead to shortages of supply.
The shortage of life-saving generic drugs is a problem that potentially could affect all of us. The solution is to deregulate and let the market work instead of piling more restrictions on an already over regulated market.

0 Comments